Open Access Article
Open Access ArticleNew facets of nanozyme activity of ceria: lipo- and phospholipoperoxidase-like behaviour of CeO2 nanoparticles†
Madina M. Sozarukova a,
Elena V. Proskurnina
a,
Elena V. Proskurnina b,
Anton L. Popov
b,
Anton L. Popov ac,
Alexander L. Kalinkin
ac,
Alexander L. Kalinkin d and
Vladimir K. Ivanov
d and
Vladimir K. Ivanov *ae
*ae
aKurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Russian Federation. E-mail: s_madinam@bk.ru; antonpopovleonid@gmail.com; van@igic.ras.ru
bResearch Centre for Medical Genetics, Russian Federation. E-mail: proskurnina@gmail.com
cInstitute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, Russian Federation
dMedical Research and Educational Center, Lomonosov Moscow State University, Russian Federation. E-mail: akalinkin@sleeplab.ru
eNational Research University Higher School of Economics, Russian Federation
First published on 2nd November 2021
Abstract
Cerium dioxide nanoparticles have a special place among engineered nanomaterials due to the wide range of their enzyme-like activities. They possess SOD-, catalase- and peroxidase-like properties, as well as recently discovered phosphatase-, photolyase-, phospholipase- and nuclease-like properties. Advancing biomedical applications of CeO2-based nanozymes requires an understanding of the features and mechanisms of the redox activity of CeO2 nanoparticles when entering the vascular bed, especially when interacting with lipid-protein supramolecular complexes (biomembranes and lipoproteins). In this paper, CeO2 nanoparticles are shown to possess two further types of nanozyme activity, namely lipo- and phospholipoperoxidase-like activities. Compared to a strong blood prooxidant, hemoglobin, CeO2 nanoparticles act as a mild oxidising agent, since they exhibit a 106 times lower, and 20 times lower, prooxidant capacity towards linoleic acid and phosphatidylcholine hydroperoxides, respectively. Compared to the widespread pharmacological preparation of iron, Fe(III) carboxymaltose (antianemic preparation Ferinject®), the prooxidant capacity of CeO2 nanoparticles towards lipid and phospholipid substrates has been shown to be 102 times lower, and 4 times higher, respectively. The data obtained on the mechanism of the interaction of nanodisperse CeO2 with the main components of biological membranes, lipids and phospholipids enable the substantial expansion of the scope of biomedical applications of CeO2 nanozymes.
Introduction
Several classes of inorganic nanomaterials, including metal oxide and noble metal nanoparticles, exhibit unexpectedly high biological activity due to their ability to mimic some natural enzymes.1,2 These materials, known as nanoenzymes or nanozymes, demonstrate relatively high stability and reproducibility of characteristics in wide temperature and pH ranges, as well as biocompatibility, the possibility of additional surface functionalisation and low cost.3–7Cerium dioxide is a striking representative of a new generation of nanozymes. The enzyme-like properties of nanocrystalline CeO2, mimicking the functions of some natural enzymes, superoxide dismutase (SOD),8–10 catalase11 and oxidase,12,13 have recently been supplemented with the discovery of new types of biochemical activity, including phosphatase-,14 photolyase-,15 phospholipase-16 and nuclease-like17 properties. This makes cerium dioxide a unique multifunctional nanozyme and expands the scope of its future biomedical applications.18–21
Recent studies have demonstrated the role of cerium dioxide as a possible regulator of free radicals in living systems. Nanocrystalline cerium dioxide has dual redox activity, combining pro- and antioxidant properties. The role of CeO2 as a free radical scavenger has been confirmed by a number of studies demonstrating inhibition of neuronal death in transgenic 5xFAD mice (Alzheimer's disease model),22 reduction of ovarian tumour growth (cancer xenograft model),23 preventing loss of photoreceptor cell function (P23H-1, the autosomal dominant retinitis pigmentosa model),24 etc.
Despite a number of sophisticated studies, little information is available on the interaction of CeO2 nanoparticles with biological molecules,25 especially with biomembranes.26,27 In eukaryotic cells, phospholipids are the predominant membrane lipids, asymmetrically embedded in a lipoprotein complex.28–30 Phospholipids are a class of lipids consisting of a glycerol backbone, a polar group and two hydrophobic acyl chains.28 In most eukaryotic membranes, phosphatidylcholine and phosphatidylethanolamine make up about 60–85% of the phospholipid fraction.28,31,32 A lipid bilayer is sensitive to free radical oxidation (primary target).33,34 Lipid peroxidation (LPO) disrupts cell membrane integrity and plays an important role in the development of cell death mechanisms, including apoptosis, oxytosis and ferroptosis.35 Since the redox activity of the CeO2 nanozyme assures both pro- and antioxidant properties, studies on cellular and animal models after exposure to CeO2 nanoparticles have shown either an increase or a decrease in the level of lipid peroxidation products, which are indicators of oxidative stress.36–41
In this study, the chemiluminescence method was used for gathering data on the redox activity of CeO2 nanoparticles, which has enabled expansion of the list of known types of enzyme-like activities of CeO2 nanoparticles, to include lipo- and phospholipoperoxidase-like activities. The enzyme-like properties of nanodisperse CeO2 and the main prooxidant of blood, hemoglobin, as well as the conventional lipid prooxidant, pharmaceutical preparation Ferinject®, which is a colloidal solution of Fe(III) carboxymaltose, were compared to reveal differences in the action mechanism of these prooxidants. The results obtained are of special importance for advancing the pharmacotherapeutic applications of CeO2 nanoparticles in the treatment of socially significant diseases.
Materials and methods
Synthesis and physicochemical study of citrate-stabilised CeO2 sol
An unstabilised colloidal solution of CeO2 nanoparticles (0.13 M) was prepared by thermohydrolysis of an aqueous solution of ammonium cerium(IV) nitrate (#215473, Sigma-Aldrich).42 Briefly, an aqueous solution of ammonium cerium(IV) nitrate (100 g L−1) was heated at 95 °C for 24 h. The precipitate formed was washed three times with isopropanol and then redispersed in deionised water and boiled for 1 h, with constant stirring, to remove isopropanol. The concentration of CeO2 sol was determined gravimetrically. CeO2 sol was further stabilised by disubstituted ammonium citrate (C6H14O7N2, #247561, Sigma-Aldrich) in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
X-ray diffraction (XRD) analysis of CeO2 samples was carried out on a Bruker D8 Advance diffractometer (CuKα radiation, geometry θ–2θ). The diffraction maxima were identified using the ICDD PDF2 databank. The average hydrodynamic diameter of CeO2 nanoparticles was estimated by dynamic light scattering (DLS), using a Photocor Complex analyser. The microstructure of the samples was studied by transmission electron microscopy (TEM) with a Leo 912 AB Omega microscope at an accelerating voltage of 100 kV.
Preparation of linoleic acid and phosphatidylcholine hydroperoxides
To prepare a phosphate buffer solution (PBS, 100 mM pH 7.4), KH2PO4 (#P0662, Sigma-Aldrich) was used. A working solution of linoleic acid (d = 0.9 g cm−3, #L1376, Sigma-Aldrich) was prepared by diluting the stock solution with dimethyl sulfoxide (≥99.95%, #472301, Sigma-Aldrich). Oxidised linoleic acid (LOOH) was obtained by air barbotage.43 The model phospholipid preparation was an ultrafine emulsion (nanoparticles up to 30 nm in size) containing 80–95% phosphatidylcholine and 20–5% maltose (Phospholipovit®, Institute of Biomedical Chemistry) dissolved in PBS (100 mM pH 7.4). Phosphatidylcholine hydroperoxide (PCOOH) was obtained by oxidation catalysed by lipoxygenase-1 (#P08170, Cayman).44The concentration of hydroperoxide groups in linoleic acid samples was determined by infrared (IR) spectroscopy.45 The height of the absorption peak at the characteristic wavelength served as the analytical signal. The concentration of linoleic acid hydroperoxide (987 cm−1, –O–O– group) in the linoleic acid samples was calculated from the calibration dependence for tert-butyl hydroperoxide (880 cm−1, –O–O– group t-BuOOH).45 The content of hydroperoxide in the samples of oxidised phosphatidylcholine was determined by HPLC with chemiluminescence detection.46
Analysis of prooxidant activity of CeO2 nanoparticles by the chemiluminescent method
Coumarin 334 (#393002, Sigma-Aldrich) was used as a chemiluminescent probe (CL probe) sensitive to lipid and phospholipid radicals. To obtain a working solution with a concentration of 5 mM, a weighed portion of coumarin 334 was dissolved in dimethyl sulfoxide (≥99.95%, #472301, Sigma-Aldrich).100 μM hemoglobin solution (deoxyhemoglobin, Hb) stabilised with lithium heparin (17 IU mL−1) was prepared by dissolving a weighed portion of lyophilised human hemoprotein powder (#H7379, Sigma-Aldrich) in distilled water in a vacuum blood collection tube (Vacutainer Plus). The exact concentration of Hb in the resulting solution was determined by optical spectroscopy, ε406 (Hb) = 270![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 548 cm−1/M.
548 cm−1/M.
For comparative evaluation of the enzyme-like activity of citrate-stabilised CeO2 sol, Ferinject® (Vifor Pharma, Fe(III) carboxymaltose), an iron-containing antianemic drug, was used. Working solutions were obtained by diluting the initial preparation (50 mg mL−1) with distilled water.
Lipo- and phospholipoperoxidase-like activity of the samples was determined by recording the kinetics of free radical oxidation, according to a previously developed technique.47 All measurements were carried out at room temperature.
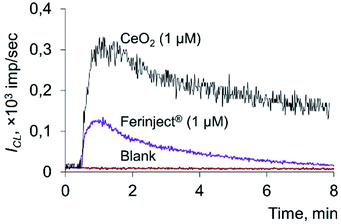
Spontaneous chemiluminescence (CL) was recorded for 30–60 s in a system containing PBS (100 mM, pH 7.4), coumarin 334 (50 μM) and model lipid or phospholipid substrates (hydroperoxides LOOH or PCOOH).48 Then, using a chromatography syringe (Hamilton), an aliquot (50 μL) of citrate-stabilised CeO2 sol or solutions of deoxyhemoglobin or Fe(III) carboxymaltose was injected without interrupting the CL recording. This procedure allowed for the rapid recording of the kinetics of peroxidation processes (Fig. 1). Registration of the kinetics was performed for 5–7 min. As an analytical signal, the light sum was used, which is the area under the CL curve over a certain time interval (4 min) and is directly proportional to the number of formed radicals.
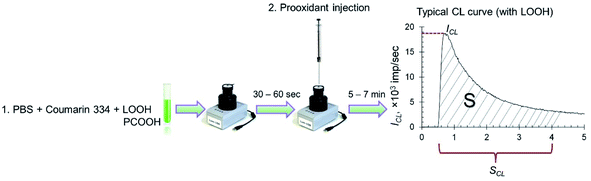 | ||
| Fig. 1 Protocol for the determination of prooxidant activity towards lipid (LOOH) and phospholipid (PCOOH) hydroperoxides by the chemiluminescent method. | ||
Results and discussion
Physicochemical characteristics of CeO2 nanoparticles
Thermohydrolysis of an aqueous solution of ammonium cerium(IV) nitrate resulted in an electrostatically stabilised sol of nanocrystalline cerium dioxide. The content of CeO2 in the sol was 23 g L−1 (0.13 M). According to XRD data (Fig. 2a) for the dried sol, it contained single-phase cerium dioxide (PDF2 34-0394). The particle size estimated by the Scherrer equation was 3 nm. The mean hydrodynamic diameter of CeO2 nanoparticles obtained by the DLS method (Fig. 2b) was found to be 10–11 nm. The data on the particle size and phase composition of the obtained material were confirmed by the results of the analysis of the CeO2 sol by TEM and electron diffraction (Fig. 2c).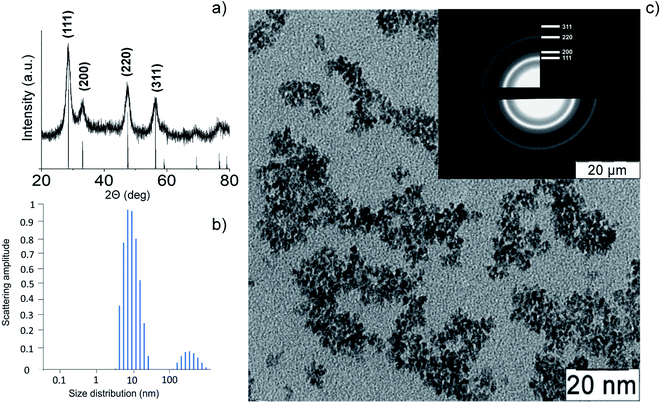 | ||
| Fig. 2 Cerium oxide nanoparticles: (a) XRD pattern, (b) dynamic light scattering pattern, (c) TEM image and electron diffraction data (inset). | ||
The electron diffraction data and XRD analysis also confirm high degree of crystallinity of CeO2 nanoparticles. The highly crystalline nature of cerium oxide is consistent with recently reported study of citrate-stabilised ceria nanoparticles.49
Lipoperoxidase-like activity of CeO2 nanoparticles
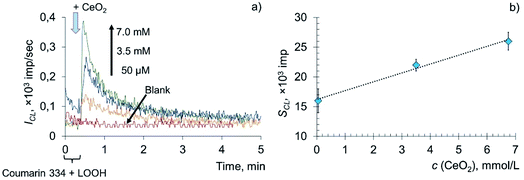
The interaction of metal oxide nanoparticles with biomembranes is of key importance when considering their biological activity.50–52 Surprisingly, information on the redox activity of CeO2 nanoparticles towards lipids and phospholipids is very scarce.25,53–55 This mainly consists of studies devoted to the interaction of CeO2 nanoparticles with model membranes, e.g. lipid films or liposomes. At the same time, the rapid expansion of potential biomedical applications of CeO2 nanoparticles makes it necessary to reveal their role in reactive oxygen species' homeostasis, including their interaction with oxidised lipoproteins of blood.The prooxidant activity of citrate-stabilised CeO2 sol was studied using the chemiluminescent method in a system containing linoleic acid hydroperoxide (LOOH) and coumarin 334, a highly selective lipid radical CL probe.56–58 The use of the probe allows for the increase in the CL quantum yield and for the detection of free radical formation. According to a previously published protocol,45 the concentration of hydroperoxide groups in LOOH samples estimated by IR spectroscopy was 100 ± 12 μmol L−1.
The addition of various concentrations of a citrate-stabilised colloidal solution of CeO2 nanoparticles to the mixed solution of LOOH and coumarin 334 resulted in a dose-dependent enhancement of chemiluminescence (Fig. 3a). The area under the curve (SCL) was chosen as a measure of prooxidant capacity proportional to the number of formed reactive oxygen species per unit concentration of the prooxidant. The dependence of the analytical signal on the concentration of CeO2 nanoparticles (Fig. 3b) was described by the equation SCL = (1.50 ± 0.29) × c (CeO2, mmol L−1) + (16 ± 2), r = 0.995 (P = 0.95, n = 3), where SCL is the area under the corresponding CL curve, ×103 imp, c (CeO2) is the concentration of CeO2 sol, mM.
The lipoperoxidase-like activity of nanodisperse cerium dioxide is demonstrated by the dose-dependent effect of chemiluminescence enhancement. A sharp increase in the intensity of chemiluminescence, followed by an exponential decay, which is characteristic of LPO, is additional evidence of lipid peroxidation induced by CeO2 nanoparticles.57,59
The data on the prooxidant activity of nanodisperse CeO2 towards linoleic acid hydroperoxide are consistent with previously reported results, demonstrating the possible induction of lipid peroxidation by CeO2 nanoparticles at the cellular and organismal levels.36,38,40 In these studies, the induction of LPO by CeO2 nanoparticles was assessed by the level of various biomarkers, such as malondialdehyde, isoprostane, etc. To describe the LPO mechanism induced by CeO2 nanoparticles (20 nm, 3.5, 10.5 and 23.3 μg mL−1) on human bronchoalveolar carcinoma cell culture (A549), the following scheme was proposed:36
| Ce4+ + Ared− → Ce3+ + Aox | (1) |
| Ce3+ + O2 → Ce4+ + O2− | (2) |
| O2− + O2− + 2H+ → O2 + H2O2 | (3) |
| H2O2 + Ce3+ → Ce4+ + OH− + OH˙ | (4) |
| LOOH + Ce3+ → Ce4+ + LO˙ + OH− | (5) |
The induction of LPO after the treatment of cell cultures with nanodisperse cerium dioxide was also observed in the models of human hepatoma (SMMC-7721, CeO2 NPs 20–30 nm; 50 mg mL−1)38 and human melanoma (A375, CeO2 NPs ∼38 nm; 20, 40, 80, 120 μg mL−1).40 Increasing the concentration of CeO2 nanoparticles led to increased levels of the main LPO marker, malondialdehyde. In addition, preliminary treatment of the cells with antioxidants such as N-acetylcysteine significantly reduced the formation of ROS and malondialdehyde and facilitated the restoration of the activity of antioxidant enzymes.38
The above in vitro studies with cancer cells demonstrated the fact that, in an acidic environment, CeO2 nanoparticles lose their antioxidant (cytoprotective) activity and begin to function as a prooxidant, causing the development of oxidative stress, including LPO, and, subsequently, apoptosis. In the current study, lipoperoxidase-like activity of CeO2 nanoparticles at pH 7.4 was observed. Analysis of existing data indicated that the redox activity of CeO2 nanoparticles represents a set of closely related pro- and antioxidant properties determined by several factors, including the pH of the reaction medium, the method of preparation and the size of synthesised nanoparticles, the nature of surface ligands, charge, etc.60 Park et al., when studying the cytotoxicity of CeO2 nanoparticles, proposed that cell type also affects the antioxidant action of ceria nanoparticles due to the factors associated with cell physiology.61 The results of the studies of CeO2 nanoparticles' interaction with malignant cells suggest oxidative stress and LPO to be the most probable mechanisms of their toxicity.62 Thus, the lipoperoxidase-like activity of CeO2 nanoparticles can be considered to be one of the key factors leading to adverse effects on the cells. The intravenous administration of an aqueous dispersion of CeO2 nanoparticles (∼31 ± 4 nm) to rats has been reported to increase levels of an LPO product, 4-hydroxy-trans-2-nonenal, the highest concentrations of which have been found in the hippocampus.39 Similarly, a high content of malondialdehyde in lung cells, indicating the toxic effect of CeO2 nanoparticles (55 nm) and leading to membrane damage, has been found in rats exposed to CeO2-containing aerosol.37 Increased LPO has been observed after intratracheal injection of a suspension of CeO2 nanoparticles (∼20 nm) to mice.41
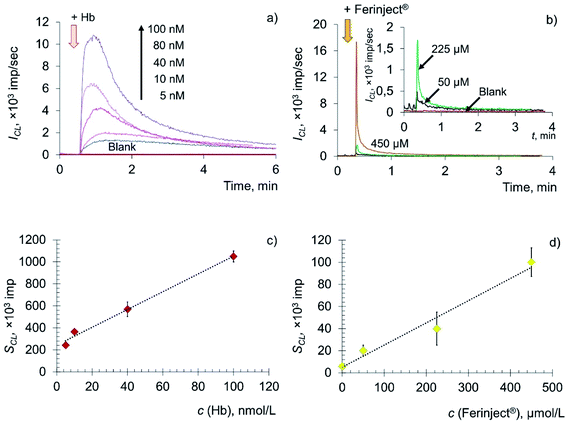
The key role in the formation of radicals in blood plasma is played by the decomposition of lipid hydroperoxides by metal ions of variable valence (primarily Fe2+) and hemoglobin. Thus, in this paper, deoxyhemoglobin (Hb), a natural ferrous compound, and Ferinject®, an antianemic drug containing Fe(III) in a stable form of a polynuclear iron hydroxide complex with a carbohydrate ligand, were chosen as model prooxidants.63–65 Experimental time dependences of chemiluminescence of the solutions containing deoxyhemoglobin and Ferinject® are shown in Fig. 4a and b, along with the corresponding concentration dependences (Fig. 4c and d).
The addition of Hb and Fe(III) carboxymaltose solutions to the system containing LOOH resulted in the rapid development of an intense chemiluminescence accompanying the lipoperoxidase-like reaction in a dose-dependent manner: SCL = (8.01 ± 0.02) × c (Hb, nM) + (249 ± 18), r = 0.996, n = 4; SCL = (0.20 ± 0.01) × c (Fe(III), μM) + (5.15 ± 0.09), r = 0.992, n = 4.
The data obtained indicate that citrate-stabilised CeO2 sol (Fig. 3a), deoxyhemoglobin (Fig. 4a) and Fe(III) carboxymaltose (Fig. 4b) solutions induced linoleic acid hydroperoxide decomposition, while catalytic activity of these compounds differed significantly. Citrate-stabilised CeO2 sol possessed significantly lower prooxidant capacity (by more than six orders of magnitude) towards linoleic acid hydroperoxide than deoxyhemoglobin. In turn, the prooxidant capacity of Ferinject® preparation was two orders of magnitude higher than that of CeO2 sol. This enables CeO2 nanoparticles to be classified as a mild prooxidant agent. A possible reason for the relatively low lipoperoxidase-like activity of citrate-stabilised CeO2 sol could be phosphate groups adsorbed on the surface of nanoparticles. This has been previously proven for other types of CeO2 enzyme-like activities, namely SOD-like and catalase-like activities.66,67 In turn, the similarity of the redox properties of citrate-stabilised CeO2 sol and Ferinject® to hydrogen peroxide (Fig. S1 in ESI†) can be partially explained by their common nature. The latter is also a colloidal solution of 20 nm nanoparticles which consists of a polynuclear iron(III)-oxyhydroxide core stabilised by carboxymaltose.64 Comparison of the kinetics of lipid hydroperoxide decomposition in the presence of CeO2 nanoparticles (Fig. 3) with previously obtained data on the CeO2-assisted decomposition kinetics of hydrogen peroxide (Fig. S2 in ESI†)68 enables the suggestion of similar mechanisms of the prooxidant activity of CeO2 nanoparticles towards different substrates.
Data on the prooxidant properties of CeO2 nanoparticles are apparently in contrast to numerous results demonstrating their cytoprotective activity.19,69,70 The inhibitory effect of CeO2 on LPO suggests the dual nature of its regulatory function. For example, the inhibition of LPO by CeO2 nanoparticles has been shown in a model of the dominant retinitis pigmentosa of rats (P23H-1, 3–5 nm CeO2 NPs; 344 ng per eye),24 in a model of hypobaric hypoxia (Sprague Dawley rat line, 7–10 nm CeO2 NPs),71 in an in vitro model of diabetic ketoacidosis (HepG2, CeO2 NPs)72 and in a study of nephrotoxicity caused by Cisplatin® (113 ± 14 nm CeO2 NPs).73
Thus, existing data make possible the suggestion that different concentrations of CeO2 nanoparticles provide either an activating or inhibiting effect on LPO. Results of the current research demonstrate that citrate-stabilised CeO2 sol, at 0.05–7.0 mM concentrations, in a model of LOOH oxidation, exhibits prooxidant properties only, being an LPO inducer. Among the factors that change the action of substances from being antioxidant to being prooxidant are the presence of other metal ions, the concentration of the substance and its redox potential.74,75 For example, Fe2+ ions can act as both pro- and antioxidants. The dual role of Fe2+ ions in chain peroxidation reactions is due to the fact that they react with both hydroperoxides and free radicals.76,77 Interacting with the former, Fe2+ ions promote branching of the lipid oxidation chains and thereby activate LPO, which in this case is a prooxidant. In turn, when reacting with free radicals, Fe2+ ions act as an antioxidant. At each site of the chain reaction, the lipoperoxyl radical leading the chain can react with either a new lipid molecule or an inhibitor, (in this case, Fe2+ ions). When the probability of the former process is higher, the chain reaction proceeds with self-acceleration; when the latter process prevails, the chain reaction proceeds with deceleration. Thus, the concentration of Fe(II) species plays a determining role in LPO induction. The similar exponential decay kinetics (and, most probably, similar mechanisms) of lipid peroxidation induced by CeO2 nanoparticles, deoxyhemoglobin (Fe(II)) and Fe(III) carboxymaltose (Ferinject®) suggest CeO2 concentration to be one of the factors determining whether there is a prooxidant or antioxidant effect of CeO2 nanoparticles on lipid peroxidation.
Phospholipoperoxidase-like activity of CeO2 nanoparticles
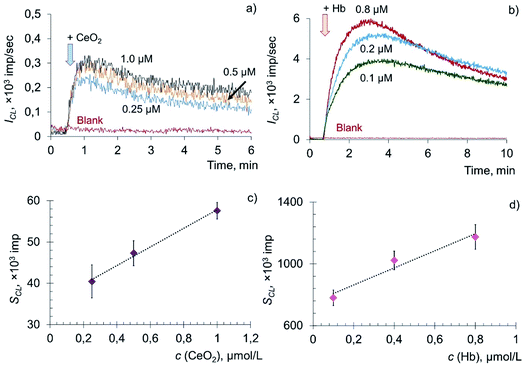
Phospholipids play multiple roles in cells. Phospholipids not only modulate membrane permeability and act as their supporting scaffold, but also participate in signal transduction in response to external and internal stimuli.28,78–80 In addition, they are the source of arachidonic acid and other polyunsaturated fatty acids, from which, as a result of a number of metabolic reactions, lipid mediators are formed that regulate various biological functions.80,81 It should be noted that phospholipids are the major targets of reactive oxygen species attack under conditions of oxidative stress.82 In this regard, the biochemical activity of CeO2 nanoparticles towards the key component of the cell membrane, phospholipids, was also investigated.The prooxidant activity of citrate-stabilised CeO2 sol was analysed by a chemiluminescence method in a biochemical model containing oxidised phosphatidylcholine (PCOOH) and coumarin 334. Amounts of hydroperoxide groups in the phospholipid substrate were determined according to an established protocol46 and were found to be 98 ± 10 μmol L−1. An aqueous solution of deoxyhemoglobin (Fe(II)) was used as a reference sample.
The addition of citrate-stabilised CeO2 sol to the solution of phosphatidylcholine hydroperoxide and coumarin 334 led to a dose-dependent increase in chemiluminescence, which reached a stationary level and then gradually decreased (Fig. 5a). The dependence of the CL signal on the concentration of CeO2 sol (Fig. 5b) was described by the equation: SCL = (22 ± 3) × c (CeO2, μM) + (35 ± 5), r = 0.997, n = 3. In a similar way, chemiluminograms were recorded for deoxyhemoglobin (Fig. 5c). A dose-dependent increase in luminescence intensity was observed (Fig. 5d): SCL = (552 ± 80) × c (Hb, μM) + (752 ± 119), r = 0.976, n = 3.
The dose-dependent enhancement of chemiluminescence caused by the addition of CeO2 nanoparticles and Hb solution to the phospholipid substrate suggests their phospholipoperoxidase-like activity. Presumably, the mechanism of interaction of Hb with PCOOH is similar to the mechanism of oxidation of hydroperoxides (LOOH) induced by metal ions of variable valence: PCOOH → PCO˙ + breakdown products.83 However, the kinetics of catalytic decomposition of PCOOH induced by CeO2 nanoparticles and Hb developed much more slowly in comparison with the oxidation of lipid hydroperoxide (LOOH).
To assess the quantitative differences in activity of CeO2 nanoparticles and Hb, the prooxidant capacity of deoxyhemoglobin was taken as 1. It was found that citrate-stabilised CeO2 sol possessed approximately 20 times less prooxidant capacity in the reaction of phosphatidylcholine hydroperoxide peroxidation than deoxyhemoglobin.
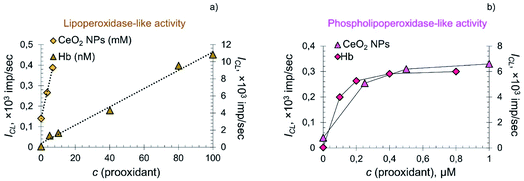
In the systems containing lipid and phospholipid hydroperoxides, CeO2 nanoparticles demonstrated significantly lower prooxidant activity compared to hemoprotein. The dependence of the CL signal on the concentration of citrate-stabilised CeO2 sol and Hb in the system containing LOOH was linear (Fig. 6a), whereas in the system containing phospholipid hydroperoxide the dependence was of a complex nature (Fig. 6b).
There are several factors that determine the switching of the lipid peroxidation process from self-accelerating to decelerating when the substrate is phosphatidylcholine hydroperoxide. Phosphatidylcholines (1,2-diacyl-sn-glycero-3-phosphocholines, lecithins) have a zwitter-ionic structure in a wide pH range.84 Important factors affecting the oxidation of phospholipids are the size and composition of liposomes, the nature of the prooxidant and the acyl chain that determines the selectivity of substrate modification.82 Recently, kinetic patterns of egg lecithin oxidation in its liposomal aqueous solutions were reported.84 It was found that the oxidation rate was proportional to the radical initiation rate and depended nonlinearly on phospholipid concentration.84 This is explained by the fact that, in a microheterogeneous environment, along with individual radicals and molecules, microaggregates (liposomes) also exist, whose reactivity differs from that of molecular species.84
In the current study, differences in the reaction kinetics for systems containing lipid and phospholipid hydroperoxides were probably due to their nature. In oil-in-water emulsions, the oxidation rate of lipid substrates is strongly influenced by the properties of the interface, determining the rate of the interaction of water-soluble prooxidants with hydroperoxides localised within, and on, the boundary of the emulsion drop.84–86 Visually, the linoleic acid hydroperoxide sample is an emulsion in a phosphate buffer solution, whereas PCOOH is a homogeneous solution formed as a result of an oxidative reaction catalysed by lipoxygenase-1 from an ultradispersed emulsion of phosphatidylcholine (with nanoparticles as small as 30 nm).
A comparison of chemiluminescence curves obtained for CeO2 nanoparticles and Fe(III) carboxymaltose (Ferinject®), in the presence of PCOOH, is provided in Fig. 7.
The prooxidant capacity of citrate-stabilised CeO2 sol was four times higher than that of a colloidal solution of Fe(III) carboxymaltose. It should be noted that the ammonium citrate did not contribute to the enhancement of chemiluminescence when CeO2 nanoparticles were added (Fig. S3 in ESI†).
To fully understand the biological activities taking place, and to expand the field of biomedical applications for CeO2 nanozymes, the mechanisms of nanoparticle–membrane interactions need to be analysed. There are only a few works devoted to the redox activity of nanodisperse CeO2 towards phospholipids. Khulbe et al. focused their attention on the phospholipase-like (phospholipase C) mimetic activity of polymer-coated CeO2 nanoparticles.16 CeO2 nanoparticles demonstrated bactericidal activity, causing membrane disruption in a wide range of pathogens, including respiratory pathogens (Klebsiella pneumoniae) and biofilm-forming bacteria.16 Important data on the molecular mechanisms of CeO2 nanoparticles' interaction with the cell membrane model were obtained by Liu et al.,53 who showed that the interaction of CeO2 nanoparticles with phosphocholine liposomes composed of two different types of phospholipid, DOPC and DPPC, proceeds mainly via the phosphate groups in the phospholipids.53
The present study demonstrates that citrate-stabilised CeO2 sol exhibits prooxidant activity towards organic hydroperoxides that were different in nature. The phospholipoperoxidase-like activity of CeO2 nanoparticles was notably higher than their lipoperoxidase-like activity (by approximately 25 times, see also ESI†). This confirms the special tropism of CeO2 nanoparticles to phospholipids. Recently, CeO2 enzyme-like activity was reported to depend strongly on particle size, most probably due to the differences in surface atoms concentration and cerium valence state.39,87,88 Generally speaking, for smaller CeO2 nanoparticles, the catalytic properties were found to be more pronounced.12,89,90 In the present study, new types of enzyme-like activities of CeO2 NPs were discovered, which are also expected to be dependent on ceria particle size in the same manner. However, the influence of the nanoparticles' size on their catalytic properties may be quite different. For example, Liu et al. showed that, especially for the peroxidase mimicking activity, smaller particles do not always exhibit higher catalytic activity.49
Temperature is an important parameter affecting the activity of enzymes, thus it was necessary to investigate the enzyme-like activities of nanocrystalline ceria at biologically relevant temperatures, too. At 37 °C, the lipo- and phospholipoperoxidase-like activities of CeO2 nanoparticles were higher than at 25 °C, by approximately 40 ± 2% (see Fig. S4 and S5 in ESI†). Interestingly, when the temperature was increased from 25 to 37 °C, lipo- and phospholipoperoxidase-like activity of deoxyhemoglobin increased to a markedly higher extent. These observations are almost in line with the milder prooxidant properties of nanoceria compared to deoxyhemoglobin and the higher sensitivity of protein catalysts to the influence of temperature than inorganic catalysts.
Analysis of the kinetics of lipid peroxidation induced by CeO2 nanoparticles, deoxyhemoglobin (Fe(II)) and Fe(III) carboxymaltose (Ferinject®) enabled the assumption of similar mechanisms in relation to their prooxidant action. That is, nanoceria-induced lipid or phospholipid peroxidation could be similar to conventional iron-assisted peroxidation (Fig. 8).57,59,77,91 CeO2 NPs catalyse oxidation of both linoleic acid and phosphatidylcholine peroxides, and free radicals are consequently formed. The key difference between these catalytic reactions are different reaction rates, phospholipid peroxidation proceeding slower than lipid peroxidation.
New types of enzyme-like activities of ceria additionally expand the scope of its practical applications. In particular, artificial enzymes possessing specific activity can be used for fine tuning enzyme-catalysed processes in living cells.
Non-enzymatic lipid oxidation is usually considered deleterious, while enzymatic peroxidation is considered beneficial for cell survival and generates relatively few lipid regulators. However, both processes often proceed simultaneously and may influence each other. Recent studies have demonstrated that lipid peroxidation products play an important role in signalling92,93 through reactive carbonyls such as malonic dialdehyde and 4-hydroxynonenal, which are able to react with proteins and modify them.94–96 Lipid aldehydes activate proinflammatory NF-κB signalling pathways.97 Non-enzymatic inducers of lipid peroxidation, such as iron or copper complexes, react with lipid hydroperoxides, phospholipid peroxides and hydrogen peroxide. Since CeO2 NPs exhibit mostly phospholipoperoxidase-like activity, they can act as a mild selective agent for phospholipid peroxidation. Such an artificial triggering of LPO-dependent signalling pathways would allow for a deeper insight into pathological processes in the cells. On the other hand, a relatively low lipoperoxidase-like activity is beneficial for the possible use of nanoceria as a theranostic agent.98,99
Conclusions
In the present study, CeO2 nanoparticles were shown to possess two novel types of enzyme-like activity, namely lipoperoxidase and phospholipoperoxidase activity. In the presence of ceria NPs, organic peroxide substrates (linoleic acid hydroperoxide and phosphatidylcholine hydroperoxide) decomposed catalytically to form free radicals. The prooxidant capacity of CeO2 was quantitatively assessed using a chemiluminescent method with a coumarin 334 luminescent probe. Lipoperoxidase-like activity of nanoceria appeared to be lower than phospholipoperoxidase-like activity, probably due to the higher tropism of ceria towards phosphate groups. Both phospholipoperoxidase-like and lipoperoxidase-like activities of nanoceria were significantly lower than that of deoxyhemoglobin. Thus, CeO2 nanoparticles can act as a mild selective agent for phospholipid peroxidation.Mild lipoperoxidase- and phospholipoperoxidase-like activities are advantageous for the possible biomedical applications of CeO2 nanoparticles as a lipid and phospholipid radical regulator in living systems.
Author contributions
Conceptualisation, E. V. P. and V. K. I.; methodology, M. M. S., E. V. P. and V. K. I.; data curation, E. V. P.; investigation, M. M. S. and A. L. P.; validation, M. M. S.; supervision, V. K. I.; funding acquisition, M. M. S.; project administration, V. K. I.; resources, A. L. K.; writing—original draft preparation, M. M. S.; writing—review and editing, E. V. P. and V. K. I. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors have no conflicts of interest to disclose.Acknowledgements
This work was supported by the Russian Science Foundation (project 21-73-00251).References
- D. Jiang, D. Ni, Z. T. Rosenkrans, P. Huang, X. Yan and W. Cai, Chem. Soc. Rev., 2019, 48, 3683–3704 RSC.
- H. Wang, K. Wan and X. Shi, Adv. Mater., 2019, 31, 1805368 CrossRef CAS PubMed.
- R. Zhang, K. Fan and X. Yan, Sci. China: Life Sci., 2020, 63, 1183–1200 CrossRef PubMed.
- T. Kang, Y. G. Kim, D. Kim and T. Hyeon, Coord. Chem. Rev., 2020, 403, 213092 CrossRef CAS.
- X. Liu, Y. Gao, R. Chandrawati and L. Hosta-Rigau, Nanoscale, 2019, 11, 21046–21060 RSC.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS PubMed.
- X. Wang, W. Guo, Y. Hu, J. Wu and H. Wei, Nanozymes: next wave of artificial enzymes, Springer International Publishing, 2016 Search PubMed.
- C. Korsvik, S. Patil, S. Seal and W. T. Self, Chem. Commun., 2007, 1056–1058 RSC.
- E. G. Heckert, A. S. Karakoti, S. Seal and W. T. Self, Biomaterials, 2008, 29, 2705–2709 CrossRef CAS PubMed.
- M. M. Sozarukova, M. A. Shestakova, M. A. Teplonogova, D. Y. Izmailov, E. V. Proskurnina and V. K. Ivanov, Russ. J. Inorg. Chem., 2020, 65, 597–605 CrossRef CAS.
- T. Pirmohamed, J. M. Dowding, S. Singh, B. Wasserman, E. Heckert, A. S. Karakoti, J. E. King, S. Seal and W. T. Self, Chem. Commun., 2010, 46, 2736–2738 RSC.
- A. Asati, S. Santra, C. Kaittanis, S. Nath and J. M. Perez, Angew. Chem., Int. Ed., 2009, 48, 2308–2312 CrossRef CAS PubMed.
- B. Liu, Z. Huang and J. Liu, Nanoscale, 2016, 8, 13562–13567 RSC.
- T. Yao, Z. Tian, Y. Zhang and Y. Qu, ACS Appl. Mater. Interfaces, 2019, 11, 195–201 CrossRef CAS PubMed.
- Z. Tian, T. Yao, C. Qu, S. Zhang, X. Li and Y. Qu, Nano Lett., 2019, 19, 8270–8277 CrossRef CAS PubMed.
- K. Khulbe, K. Karmakar, S. Ghosh, K. Chandra, D. Chakravortty and G. Mugesh, ACS Appl. Bio Mater., 2020, 1–36 Search PubMed.
- F. Xu, Q. Lu, P. J. Huang and J. Liu, Chem. Commun., 2019, 55, 13215–13218 RSC.
- A. B. Shcherbakov, V. V. Reukov, A. V. Yakimansky, E. L. Krasnopeeva, O. S. Ivanova, A. L. Popov and V. K. Ivanov, Polymers, 2021, 13, 924 CrossRef CAS PubMed.
- A. B. Shcherbakov, N. M. Zholobak and V. K. Ivanov, in Cerium Oxide (CeO2): Synthesis, Properties and Applications, ed. S. Scirè and L. Palmisano, Elsevier, Amsterdam, 2020, pp. 279–358 Search PubMed.
- S. Rajeshkumar and P. Naik, Biotechnol. Rep., 2018, 17, 1–5 CrossRef CAS PubMed.
- A. Dhall and W. Self, Antioxidants, 2018, 7, 97 CrossRef PubMed.
- H. J. Kwon, M.-Y. Cha, D. Kim, D. K. Kim, M. Soh, K. Shin, T. Hyeon and I. Mook-Jung, ACS Nano, 2016, 10, 2860–2870 CrossRef CAS PubMed.
- M. Hijaz, S. Das, I. Mert, A. Gupta, Z. Al-Wahab, C. Tebbe, S. Dar, J. Chhina, S. Giri and A. Munkarah, BMC Cancer, 2016, 16, 1–14 CrossRef PubMed.
- L. L. Wong, Q. N. Pye, L. Chen, S. Seal and J. F. McGinnis, PLoS One, 2015, 10, e0121977 CrossRef PubMed.
- A. Kumar, S. Das, P. Munusamy, W. Self, D. R. Baer, D. C. Sayle and S. Seal, Environ. Sci.: Nano, 2014, 1, 516–532 RSC.
- T. Xia, M. Kovochich, M. Liong, L. Madler, B. Gilbert, H. Shi, J. I. Yeh, J. I. Zink and A. E. Nel, ACS Nano, 2008, 2, 2121–2134 CrossRef CAS PubMed.
- Z. Ji, X. Wang, H. Zhang, S. Lin, H. Meng, B. Sun, S. George, T. Xia, A. E. Nel and J. I. Zink, ACS Nano, 2012, 6, 5366–5380 CrossRef CAS PubMed.
- R. Pamplona, Biochim. Biophys. Acta, Bioenerg., 2008, 1777, 1249–1262 CrossRef CAS PubMed.
- M. Ikeda, A. Kihara and Y. Igarashi, Biol. Pharm. Bull., 2006, 29, 1542–1546 CrossRef CAS PubMed.
- G. Lenoir, P. Williamson and J. C. Holthuis, Curr. Opin. Chem. Biol., 2007, 11, 654–661 CrossRef CAS PubMed.
- M. Portero-Otín, M. J. Bellumunt, M. C. Ruiz, G. Barja and R. Pamplona, Lipids, 2001, 36, 491–498 CrossRef PubMed.
- G. Van Meer, D. R. Voelker and G. W. Feigenson, Nat. Rev. Mol. Cell Biol., 2008, 9, 112–124 CrossRef CAS PubMed.
- E. Niki, Biofactors, 2008, 34, 171–180 CrossRef CAS PubMed.
- H. Yin, L. Xu and N. A. Porter, Chem. Rev., 2011, 111, 5944–5972 CrossRef CAS PubMed.
- L. J. Su, J. H. Zhang, H. Gomez, R. Murugan, X. Hong, D. Xu, F. Jiang and Z. Y. Peng, Oxid. Med. Cell. Longevity, 2019, 2019, 5080843 Search PubMed.
- W. Lin, Y. W. Huang, X. D. Zhou and Y. Ma, Int. J. Toxicol., 2006, 25, 451–457 CrossRef CAS PubMed.
- A. Srinivas, P. J. Rao, G. Selvam, P. B. Murthy and P. N. Reddy, Toxicol. Lett., 2011, 205, 105–115 CrossRef CAS PubMed.
- G. Cheng, W. Guo, L. Han, E. Chen, L. Kong, L. Wang, W. Ai, N. Song, H. Li and H. Chen, Toxicol. In Vitro, 2013, 27, 1082–1088 CrossRef CAS PubMed.
- R. A. Yokel, M. T. Tseng, M. Dan, J. M. Unrine, U. M. Graham, P. Wu and E. A. Grulke, Nanomedicine, 2013, 9, 398–407 CrossRef CAS PubMed.
- D. Ali, S. Alarifi, S. Alkahtani, A. A. AlKahtane and A. Almalik, Cell Biochem. Biophys., 2015, 71, 1643–1651 CrossRef CAS PubMed.
- A. Nemmar, P. Yuvaraju, S. Beegam, M. A. Fahim and B. H. Ali, Oxid. Med. Cell. Longevity, 2017, 2017, 9639035 Search PubMed.
- A. B. Shcherbakov, M. A. Teplonogova, O. S. Ivanova, T. O. Shekunova, I. V. Ivonin, A. E. Baranchikov and V. K. Ivanov, Mater. Res. Express, 2017, 055008 CrossRef.
- S. Adachi, T. Ishiguro and R. Matsuno, J. Am. Oil Chem. Soc., 1995, 72, 547 CrossRef CAS.
- J. Ito, K. Nakagawa, S. Kato, T. Hirokawa, S. Kuwahara, T. Nagai and T. Miyazawa, J. Chromatogr. A, 2015, 1386, 53–61 CrossRef CAS PubMed.
- A. A. Dzhatdoeva, A. M. Polimova, E. V. Proskurnina, M. A. Proskurnin and Y. A. Vladimirov, J. Anal. Chem., 2016, 71, 542–548 CrossRef CAS.
- T. Miyazawa, T. Suzuki, K. Fujimoto and K. Yasuda, J. Lipid Res., 1992, 33, 1051–1059 CrossRef CAS.
- E. V. Proskurnina, A. A. Dzhatdoeva, E. S. Lobichenko, R. I. Shalina and Y. A. Vladimirov, J. Anal. Chem., 2017, 72, 751–755 CrossRef CAS.
- P. O. Volkova, A. V. Alekseev, A. A. Dzhatdoeva, E. V. Proskurnina and Y. A. Vladimirov, Moscow Univ. Chem. Bull., 2016, 71, 87–96 CrossRef.
- X. Liu, J. Wu, Q. Liu, A. Lin, S. Li, Y. Zhang, Q. Wang, T. Li, X. An and Z. Zhou, J. Mater. Chem. B, 2021, 9, 7238–7245 RSC.
- A. E. Nel, L. Mädler, D. Velegol, T. Xia, E. M. Hoek, P. Somasundaran, F. Klaessig, V. Castranova and M. Thompson, Nat. Mater., 2009, 8, 543–557 CrossRef CAS PubMed.
- A. Verma and F. Stellacci, Small, 2010, 6, 12–21 CrossRef CAS PubMed.
- J. Liu, Langmuir, 2016, 32, 4393–4404 CrossRef CAS PubMed.
- Y. Liu and J. Liu, Langmuir, 2016, 32, 13276–13283 CrossRef CAS PubMed.
- B. Foster, M. Larios and V. Smith, FASEB J., 2016, 30, lb74 Search PubMed.
- M. H. Wong, R. P. Misra, J. P. Giraldo, S.-Y. Kwak, Y. Son, M. P. Landry, J. W. Swan, D. Blankschtein and M. S. Strano, Nano Lett., 2016, 16, 1161–1172 CrossRef CAS PubMed.
- Y. A. Vladimirov, V. S. Sharov, E. S. Driomina, A. V. Reznitchenko and S. B. Gashev, Free Radical Biol. Med., 1995, 18, 739–745 CrossRef CAS PubMed.
- Y. A. Vladimirov, V. S. Sharov, E. S. Driomina, A. V. Reznitchenko and S. B. Gashev, Free Radical Biol. Med., 1995, 18, 739–745 CrossRef CAS PubMed.
- N. Baker, G. Greenway, R. Wheatley and C. Wiles, Analyst, 2007, 132, 104–106 RSC.
- Y. A. Vladimirov, E. V. Proskurina and D. Yu. Izmailov, Biofizika, 2011, 56, 1081–1090 CAS.
- A. Asati, S. Santra, C. Kaittanis and J. M. Perez, ACS Nano, 2010, 4, 5321–5331 CrossRef CAS PubMed.
- E. J. Park, J. Choi, Y. K. Park and K. Park, Toxicology, 2008, 245, 90–100 CrossRef CAS PubMed.
- A. Nel, T. Xia, L. Madler and N. Li, Science, 2006, 311, 622–627 CrossRef CAS PubMed.
- R. A. Moore, H. Gaskell, P. Rose and J. Allan, BMC Blood Disord., 2011, 11, 4 CrossRef CAS PubMed.
- L. J. Scott, Drugs, 2018, 78, 479–493 CrossRef CAS PubMed.
- B. Dalzon, A. Torres, S. Reymond, B. Gallet, F. Saint-Antonin, V. Collin-Faure, C. Moriscot, D. Fenel, G. Schoehn, C. Aude-Garcia and T. Rabilloud, Nanomaterials, 2020, 10, 266 CrossRef CAS PubMed.
- R. N. McCormack, P. Mendez, S. Barkam, C. J. Neal, S. Das and S. Seal, J. Phys. Chem. C, 2014, 118, 18992–19006 CrossRef CAS.
- Y. Zhao, H. Li, A. Lopez, H. Su and J. Liu, ChemBioChem, 2020, 21, 2178–2186 CrossRef CAS PubMed.
- M. M. Sozarukova, E. V. Proskurnina, A. E. Baranchikov and V. K. Ivanov, Nanosyst.: Phys., Chem., Math., 2020, 11, 324–332 CAS.
- B. C. Nelson, M. E. Johnson, M. L. Walker, K. R. Riley and C. M. Sims, Antioxidants, 2016, 5, 15 CrossRef PubMed.
- R. Zhang, K. Fan and X. Yan, in Nanozymology: Connecting Biology and Nanotechnology, ed. X. Yan, Springer, 2020, ch. 9, pp. 279–329 Search PubMed.
- A. Arya, N. K. Sethy, S. K. Singh, M. Das and K. Bhargava, Int. J. Nanomed., 2013, 8, 4507–4520 Search PubMed.
- M. Shokrzadeh, H. Abdi, A. Asadollah-Pour and F. Shaki, Cell J., 2016, 18, 97–102 Search PubMed.
- M. A. Saifi, S. Sangomla, A. Khurana and C. Godugu, Biol. Trace Elem. Res., 2019, 189, 145–156 CrossRef CAS PubMed.
- B. Poljsak and P. Raspor, J. Appl. Toxicol., 2008, 28, 183–188 CrossRef CAS PubMed.
- R. Sotler, B. Poljsak, R. Dahmane, T. Jukic, D. Pavan Jukic, C. Rotim, P. Trebse and A. Starc, Acta Clin. Croat., 2019, 58, 726–736 Search PubMed.
- T. B. Suslova, V. I. Olenev, M. V. Korchagina and I. A. Vladimirov, Biofizika, 1970, 15, 622–628 CAS.
- Y. A. Vladimirov and A. A. Archakov, Lipid Peroxidation in Biological Membranes [in Russian], Nauka, Moscow, 1972 Search PubMed.
- W. Dowhan, J. Biol. Chem., 2017, 292, 10755–10766 CrossRef CAS PubMed.
- E. M. Mejia and G. M. Hatch, J. Bioenerg. Biomembr., 2016, 48, 99–112 CrossRef CAS PubMed.
- C. Solís-Calero, J. Ortega-Castro, J. Frau and F. Muñoz, Oxid. Med. Cell. Longevity, 2015, 2015, 319505 Search PubMed.
- H. Tallima and R. El Ridi, J. Adv. Res., 2018, 11, 33–41 CrossRef CAS PubMed.
- A. Reis and C. M. Spickett, Biochim. Biophys. Acta, 2012, 1818, 2374–2387 CrossRef CAS PubMed.
- Y. Bao and G. Williamson, Redox Rep., 1997, 3, 325–330 CrossRef CAS PubMed.
- E. A. Mengele, I. G. Plashchina and O. T. Kasaikina, Colloid J., 2011, 73, 815–821 CrossRef CAS.
- W. Chaiyasit, M. Silvestre, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2000, 48, 3077–3080 CrossRef CAS PubMed.
- U. Klinkesorn, P. Sophanodora, P. Chinachoti, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2005, 53, 4561–4566 CrossRef CAS PubMed.
- J. Gagnon and K. M. Fromm, Eur. J. Inorg. Chem., 2015, 2015, 4510–4517 CrossRef CAS.
- G. Song, N. Cheng, J. Zhang, H. Huang, Y. Yuan, X. He, Y. Luo and K. Huang, Catalysts, 2021, 11, 1123 CrossRef CAS.
- S. S. Lee, W. Song, M. Cho, H. L. Puppala, P. Nguyen, H. Zhu, L. Segatori and V. L. Colvin, ACS Nano, 2013, 7, 9693–9703 CrossRef CAS PubMed.
- V. Baldim, F. Bedioui, N. Mignet, I. Margaill and J.-F. Berret, Nanoscale, 2018, 10, 6971–6980 RSC.
- Y. A. Vladimirov, V. I. Olenev, T. B. Suslova and Z. P. Cheremisina, Adv. Lipid Res., 1980, 17, 173–249 CrossRef CAS PubMed.
- S. Albadri, F. Naso, M. Thauvin, C. Gauron, C. Parolin, K. Duroure, J. Vougny, J. Fiori, C. Boga, S. Vriz, N. Calonghi and F. Del Bene, Dev. Cell, 2019, 50, 73–89 CrossRef CAS PubMed.
- C. Kruger, S. J. Burke, J. J. Collier, T. T. Nguyen, J. M. Salbaum and K. Stadler, Redox Biol., 2018, 16, 248–254 CrossRef CAS PubMed.
- A. Ayala, M. F. Munoz and S. Arguelles, Oxid. Med. Cell. Longevity, 2014, 2014, 360438 Search PubMed.
- T. D. Calamaras, C. Lee, F. Lan, Y. Ido, D. A. Siwik and W. S. Colucci, Free Radical Biol. Med., 2015, 82, 137–146 CrossRef CAS PubMed.
- Y. Yang, R. Sharma, A. Sharma, S. Awasthi and Y. C. Awasthi, Acta Biochim. Pol., 2003, 50, 319–336 CrossRef CAS PubMed.
- U. C. Yadav and K. V. Ramana, Oxid. Med. Cell. Longevity, 2013, 2013, 690545 Search PubMed.
- P. Eriksson, A. A. Tal, A. Skallberg, C. Brommesson, Z. Hu, R. D. Boyd, W. Olovsson, N. Fairley, I. A. Abrikosov and X. Zhang, Sci. Rep., 2018, 8, 1–12 Search PubMed.
- J. Xie, S. Lee and X. Chen, Adv. Drug Delivery Rev., 2010, 62, 1064–1079 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06730c |
| This journal is © The Royal Society of Chemistry 2021 |